HAHN Automation Group Improves Quality and Efficiency
A leading medical manufacturer once required 20 full-time operators for a single application. We designed a solution that allowed their workforce to focus on other applications by automating the weighing of drug coating on their drug-eluting stents.
#RFID #MedTech #Stent #DrugDelivery #Traceability
- Increased traceability with RFID capability
- Manual to fully-automated system design
- Increased efficiency by 87.5%
Challenge
Like many manufacturers, they coat their stents with a drug, creating what are known as drugeluting stents (DES), to address a critical problem in cardiovascular interventions: restenosis. Prior to our solution, they were using 10 lines to produce this stent. Each line required 2 operators—meaning the team needed 20 full-time staff. The customer wanted to reduce the number of operators needed in order to reassign those operators to other tasks that require manual interaction.
Additionally, the tolerance for error in the coating process is extremely small because the amount of drug applied to the stent is critical both for its effectiveness and EMA/FDA requirements/regulations.
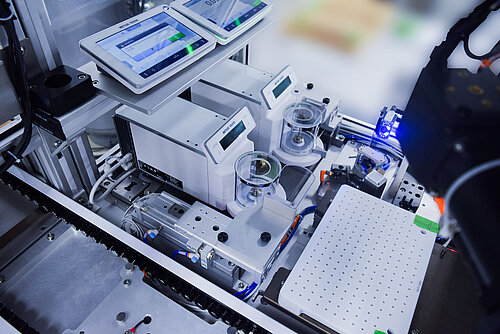
Developing a Solution
Before automation, the customer was manually loading the stents onto a scale, recording the pre-coating weight, and then inserting individual stents into equipment for the drug coating process. The stents would then be unloaded and re-weighed on the outfeed to track how much medicine was applied.
This manual operation required a full-time operator on both the infeed and outfeed of each of the 10 manufacturing lines, for a total of 20 operators. The manual tracking and weighing process was also complex due to curing time within the equipment, making it difficult to ensure the correct stent was being recorded.
Our solution included automating the infeed and outfeed of the stent manufacturing process to increase efficiency and traceability. We also used pre-loaded trays of RFID-enabled mandrels and unprocessed stents to automate the loading and tracking process.